Describe the relative densities of the phases for most substances. 350 mole Na X 6 mol NaF 4 mol Na 525 mol NaF Propane C3H8 is a common fuel used in heating homes in rural areas.
Solved Describe The Relative Densities Of The Phases For Chegg Com
Unlike most substances water is denser as a liquid than as a solid.

. Relative density or specific gravity is the ratio of the density mass of a unit volume of a substance to the density of a given reference material. The atoms are closely spaced and hence the density is high. A sealed vessel contains only water and water vapor.
However WATER is the exception to this rule. Shape Volume Answer Bank Solid Liquid ied variable Gas Question thumb_up 100. What is the relative density of the substances given this arrangement.
Na2SiF6 s 4Na s Si s 6NaF s How many moles of NaF will be produced if 350 moles of Na is reacted with excess Na2SiF6. For example 1 m 3 of lead weighs differently from 1 m 3 of wood. Most materials are more dense as solids.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. Helpful 0 Not Helpful 0. Density of gas phase.
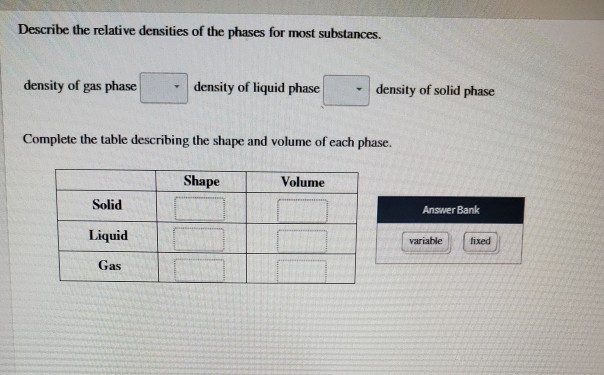
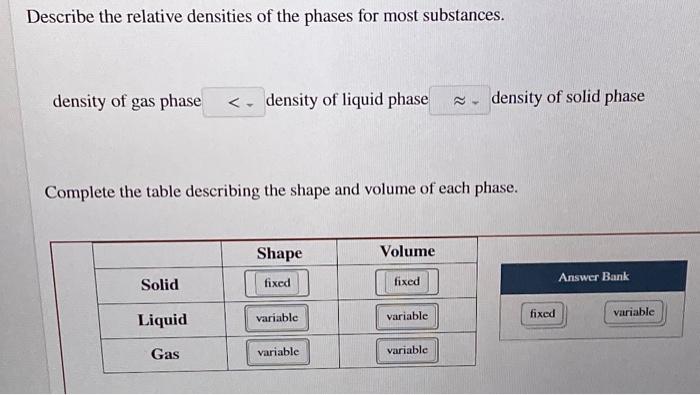
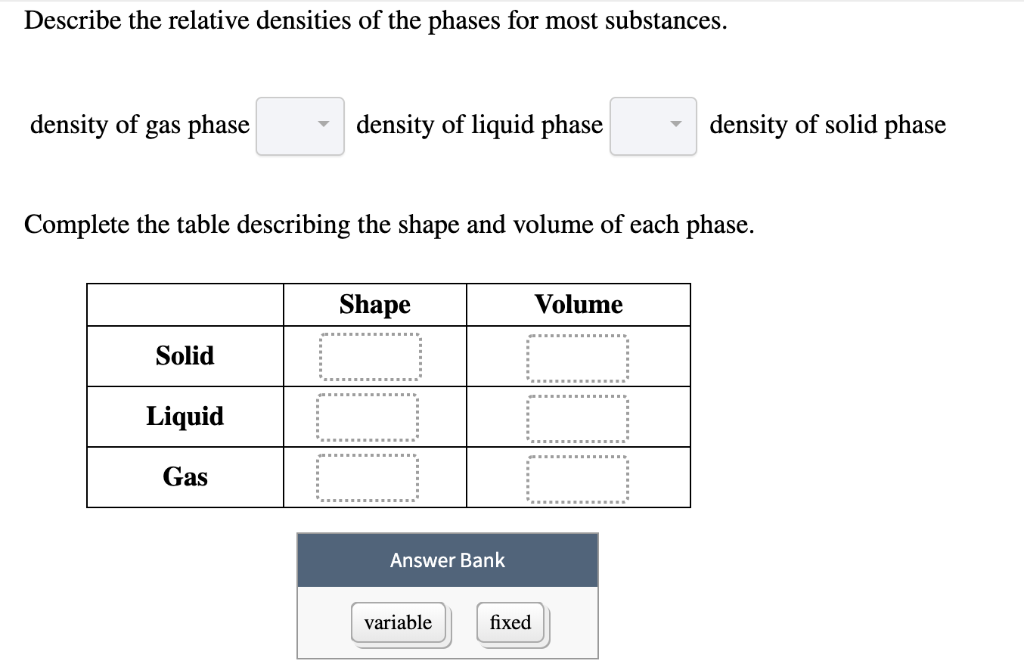
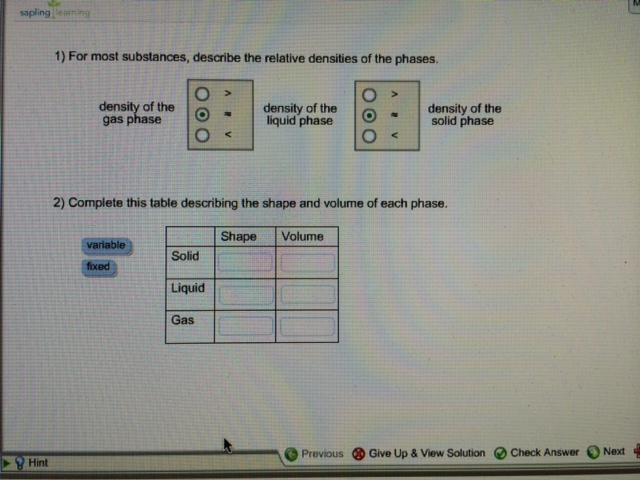
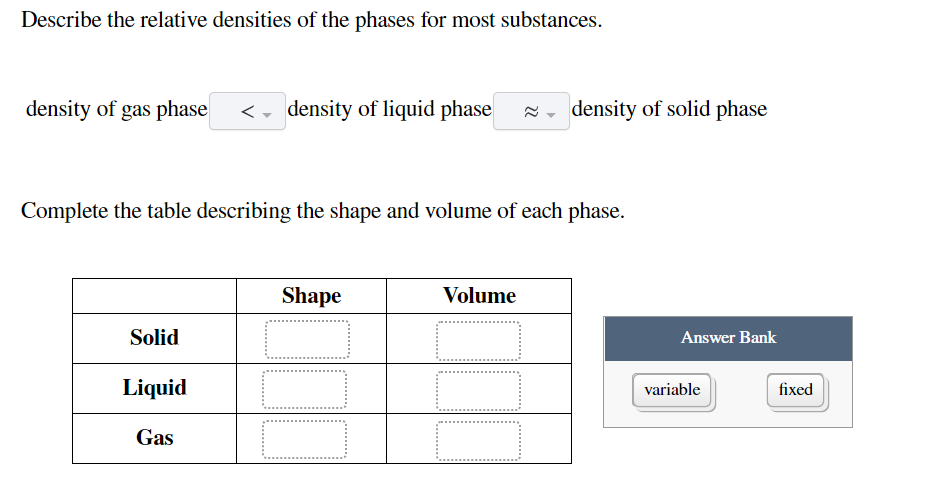
Most substances are densest as a solid because when they are cooled their particles contract. 1 For most substances describe the relative densities of the phases. 31 rows Note that the density of pure water is defined to be 1 gram per cubic centimeter or gml.
Vapor pressure is the point when evaporation and condensation of a substance reach equilibrium. Classify these images as representing a solid liquid or gas at the molecular level. Density of gas phase - density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase.
For gases the reference is air at room temperature 20 C or 68 F. The substances are red clear and blue. Also 1 m 3 of water has a weight different from that of sulphuric acid or palm oil of the same.
Shape Volume Solid Answer Bank Liquid variable Gas Mar 12 2022 0739 AM Experts Answer Solutionpdf Next Previous. 1 For most substances describe the relative densities of the phases. Notice that the triple point is well above 1 atm indicating that carbon dioxide.
Equal volumes of different substances have different masses or weights. Density of gas phase density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase. Physics SS 1 Week 8.
Also pure water is less dense than seawater so fresh water can float on top of salt water mixing at the interface. Read also relative and learn more manual guide in describe the relative densities of the phases for most substances 8 density of the gas phase density of the o density of the O solid phase uid phase 2 Complete this table describing the shape and volume of each phase. Under normal ambient conditions water is less dense as a solid than as a liquid so ice floats on water.
Density of gas phase is. When water freezes the molecules do not stack into a close-packed structure. Terms in this set 8 Describe the relative densities of the phases for most substances.
Specific gravity for liquids is nearly always measured with respect to water at its densest at 5 C or 410 F. Describe the relative densities of the phases for most substances. The density is therefore less than a solid.
Density of gas phase ____ density of liquid phase ____ density of solid phase Density of gas phase __. Rosario places the three substances in a graduated cylinder as shown. Density of gas phase.
For most substances describe the relative densities of. If its value is less than 1 then the substance is less dense than water and would float. Definition and ExperimentDetermination of solid and liquid.
Describe the relative densities of the phases for most substances. Chapters 10 and 11 Terms in this set 93 Describe the relative densities of the phases for most substances density of gas phase __ density of liquid phase__density of solid phase Complete the table describing the shape and volume of each shape Arrange the molecules by strength of the London dispersion force interactions between molecules. See answer 1 Best Answer.
Consider the following balanced equation. The solid-liquid curve exhibits a positive slope indicating that the melting point for CO 2 increases with pressure as it does for most substances water being a notable exception as described previously. 2 Complete this table describing the shape and volume of each phase.
It is also known as specific gravity SG. . Describe how the number of molecules of water in the liquid and gas phases is related to equilibrium vapor pressure.
We start with 350 mol Na. In a liquid atoms are more loosely arranged in no definite crystal arrangement and kept together by weak intermolecular forces. Relative density RD is the ratio of the density of a substance to the density of water.
Shape Volume Solid fixed fixed Answer Bank variable Liquid variable fixed variable Gas variable variable This problem has been solved. The water molecules continue to evaporate and condense in the same proportions. General Chemistry II - Chapter 11 HW Edgar Venzor 1.
Rosarios science teacher gives her three liquid substances. There are three main phases of matter. Water reaches its greatest density at 398 degrees.
Solids consist of atoms that are arranged in a regular crystal lattice. Because it is a ratio relative density or specific gravity is a unitless value. Density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase.
A consequence is that ice floats on water. .
Solved Describe The Relative Densities Of The Phases For Chegg Com
Solved Describe The Relative Densities Of The Phases For Chegg Com
Solved For Most Substances Describe The Relative Densities Chegg Com
0 Comments